BASIC SCIENTIFIC DEFINITIONS Processes that change the state or composition of matter are inevitably accompanied by the consumption or production of energy. Common processes such as combustion produce energy by the chemical rearrangement of atoms or molecules. For example, the combustion of methane (natural gas) is represented by the following reaction: CH(4) + 2 O(2) = CO(2) + ENERGY In this example, the energy produced is 8 electron volts (eV). The electron volt is a unit of energy used by nuclear physicists and represents the gain in kinetic energy when an electron is accelerated by a drop in potential by one volt. The best-known nuclear reaction is fission, in which a heavy nucleus combines with a neutron and separates into two lighter nuclei. A typical fission reaction involving uranium-235 is: 92 U235 + 1 NEUTRON = 38 Sr96 + 54 XE138 + 2 NEUTRONS + ENERGY
where the energy released is approximately 200 million electron volts (eV), a factor of 25 million times greater than that of the methane combustion reaction. Another important nuclear reaction is fusion, in which two light elements combine to form a heavier atom. An important reaction is: 1H(2) + 1 H(3) = 2 He(4) + 1 NEUTRON + ENERGY where the energy released by the reaction is 18 million eV. Nuclear fusion is a process of producing energy from the nucleus of an atom. This phenomenon occurs naturally inside the Sun and stars. Light nuclei such as hydrogen and its isotopes, deuterium and tritium, fuse and create elements of a heavier nucleus, such as helium. Nuclear (power) plants harness the enormous energy released by nuclear reactions for large-scale energy production. In a modern coal plant, the combustion of one pound (453.59g) of coal produces 1 kilowatt hour (Kwh) of electrical energy. The fission of one pound of uranium in a modern nuclear power plant produces about 3 million KWh of electrical energy. It is the incredible energy density (energy per unit mass) that makes nuclear energy sources so interesting. At present, only the fission process is used in commercial energy production (generally to produce electricity). Fusion research has not yet produced a feasible (practical) energy production technology.
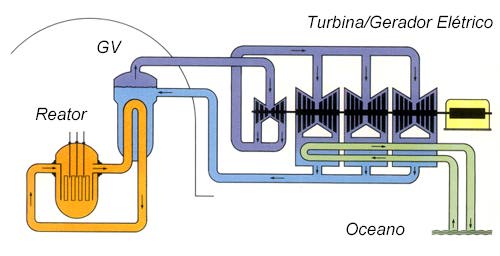
In nuclear reactors, on the contrary, energy is released at speeds that can be modified by the intervention of regulatory elements provided for there. The highest reaction speeds put into play so far, in experimental batteries, release energy at the rate of 30,000 kilowatts (Brookhaven reactor, Long Island, USA), which corresponds to the consumption of 36.2g of U-235 per day, or 13.2g/year. The atomic submarine "Nautilus" is equipped with a 60,000 kilowatt reactor, twice the power reported in these latest figures. Problem number 1 in building a reactor is obtaining nuclear "fuel", the choice of which must follow complex criteria, balancing technical and economic reasons, with restrictions of strategic origin (the danger of its diversion for war purposes). . The moderator and, possibly, the reflector must be associated with the nuclear fuel. Achieving these may be more or less difficult, depending on the substance you prefer: common water, heavy water, light water, beryllium or its oxide, graphite. In some reactors, uranium (or another active element) remains in a very fine solution or suspension inside the moderator; we have a "homogeneous" system. More frequently, however, the reactor is of the "heterogeneous" type: moderator and fissile material are in the form of bars or blocks that alternate, forming meshes of specially calculated dimensions. In any type, this core (moderator and active matter) must contain several channels, some for the insertion of other bodies, others for the passage of control bars, and still others for housing pipes through which the "refrigerant", a fluid substance, circulates. (air, water, mercury, molten metals: sodium, potassium, lead, bismuth) designed to extract from the mass the heat developed there by nuclear fission reactions and consequent radiation. Of course, all parts must be held in certain positions, and observation instruments, control bars and other accessories are necessary. Hence the use of so-called "structure" materials, which form the skeleton of the cell or house the fissionable elements and, progressively, the products resulting from nuclear fissions. All structural parts must be well resistant to radiation and operating temperatures, without hindering or capturing the passage of neutrons, which ultimately constitute particles of decisive importance for the functioning of the system.
















